- Hydrogen Deuterium Singly Ionized Helium Cell
- Hydrogen Deuterium Singly Ionized Helium Energy Level
- Hydrogen Deuterium Singly Ionized Helium Filter
- Hydrogen Deuterium Singly Ionized Helium Chemical
- Energy levels of the hydrogen atom according to Dirac theory showing the component transitions of Ha line. Hydrogen (and later deuterium) and singly ionized helium. For the sake of brevity the discussion here will be limited to the Ha (and D,) lines emitted in the transition n = 3 to n = 2.
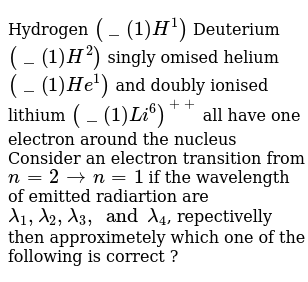
- Hydrogen (H), deuterium (D), singly ionized helium (Het) and doubly ionized lithium (Li) all have one electron around the nucleus. Consider n=2 to n=1 transition. The wavelengths of emitted radiations are 2, 1, 2, and 2, respectively.
- A neutron of kinetic energy 65 eV collides inelastically with a singly ionised helium atom at rest. It is scattered at an angle with respect to original direction. If the energy of scattered neutron is 6.36 eV, find the frequency of emitted radiation from the helium atom after suffering collision.
Hydrogen (H), deuterium (H), singly ionised helium (He) + and doubly ionised lithium (Li) all have one electron around their nucleus. Consider an electron transition from n = 2 to n = 1 if the wavelengths of the emitted radiations are λ 1, λ 2, λ 3 and λ 4 respectively then.
In which of the following systems will the wavelength corresponding to n = 2 to n = 1 be minimum?

Options
Hydrogen Deuterium Singly Ionized Helium Cell
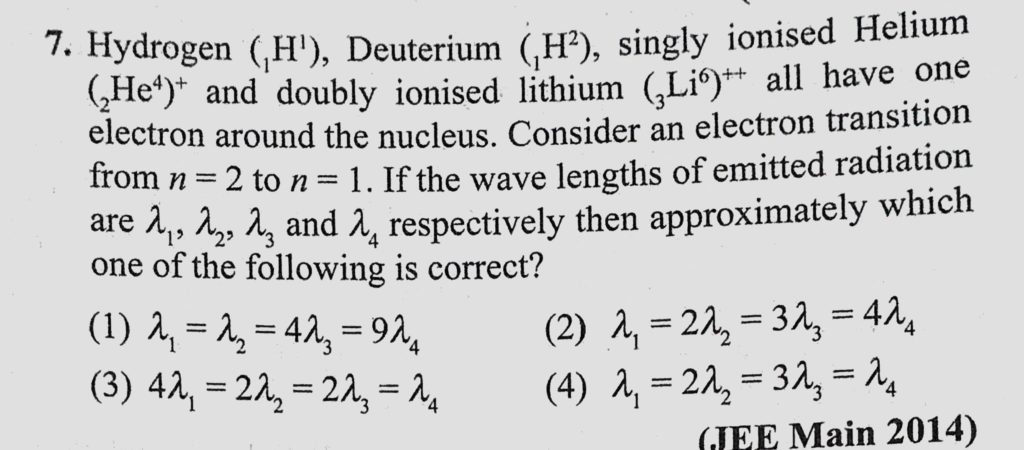
Hydrogen atom
Deuterium atom
Singly ionized helium
Doubly ionized lithium
Solution
Hydrogen Deuterium Singly Ionized Helium Energy Level
Doubly ionized lithium
The wavelength corresponding the transition fromn2 to n1 is given by Daewoo laptops & desktops driver. A4tech input devices driver download.
`1/lamda =RZ^2 (1/n_1^2 - 1/n_2^2)`]
Here,
R = Rydberg constant
Z = Atomic number of the ion
Download acpi motherboards driver. From the given formula, it can be observed that the wavelength is inversely proportional to the square of the atomic number.
Therefore, the wavelength corresponding to n = 2 to n = 1 will be minimum in doubly ionized lithium ion because for lithium, Z = 3.
Hydrogen Deuterium Singly Ionized Helium Filter

Hydrogen Deuterium Singly Ionized Helium Chemical
Video TutorialsVIEW ALL [2]
view
Video Tutorials For All Subjects
